Trends in the Next Generation Nicotine Product Industry: 2022
Innovate to accelerate. Blue-sky thinking hub.
Pharmaceuticals
Mar 30, 2021 | Published by Dr. Nveed Chaudhary
Pharmaceuticals, Nicotine
Broughton Chief Regulatory Officer, Dr Nveed Chaudhary announces the launch of our Blue-sky thinking hub aimed at nicotine, pharmaceutical, and cannabis industries.
Nveed discusses the importance of accelerating next generation products to market through sustainable partnerships that help clients turn their innovations into market value.
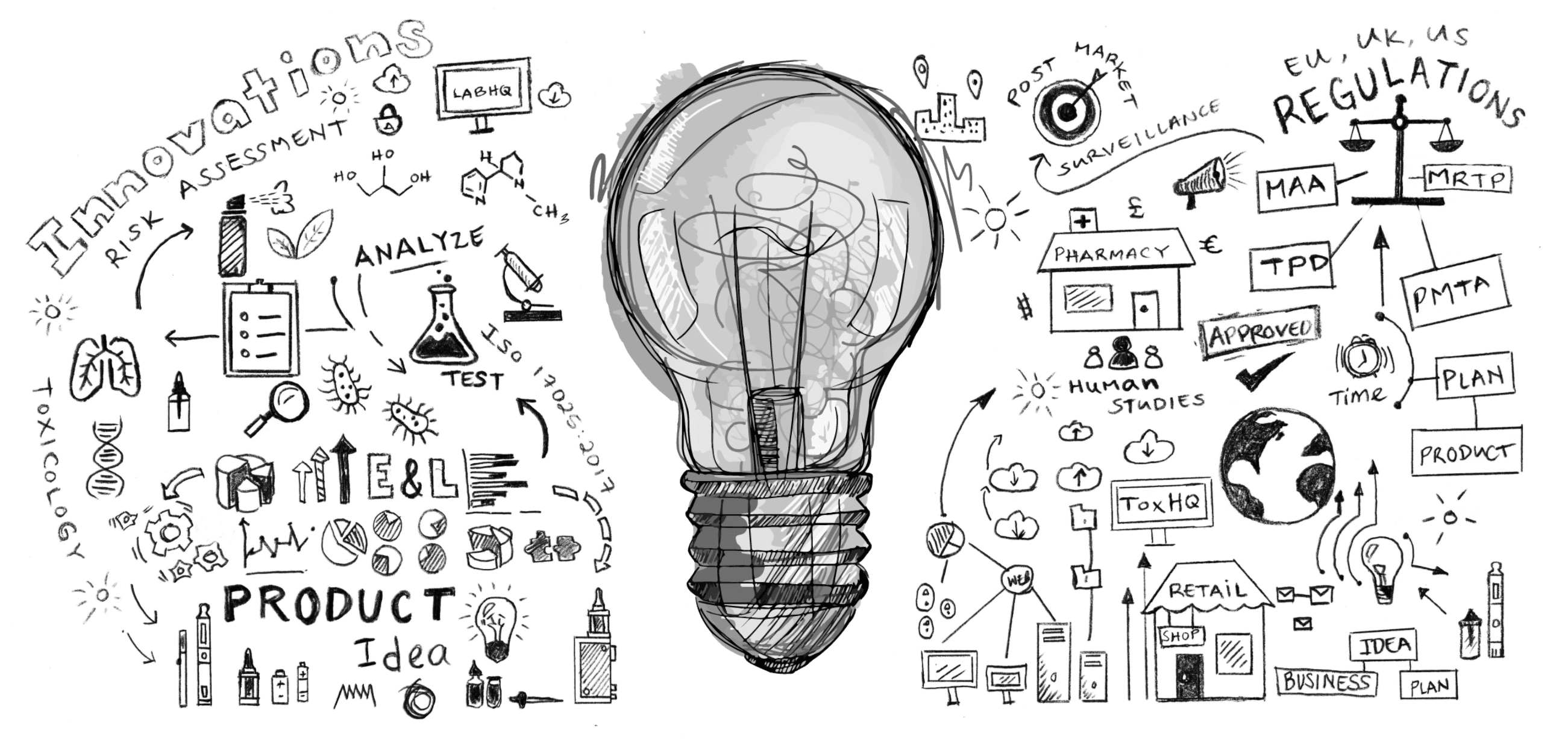
Innovation to market value
We are excited to announce the launch of our new Blue-sky thinking hub. At Broughton, we like to think differently. Like science, the world is ever changing and evolving and at the heart of this evolution lies innovation. To achieve total harm reduction, helping smokers to quit while protecting our planet and future generations, manufacturers need to work with innovative partners to meet regulatory obligations, help shape future regulation and deliver future market value. Whether advancing existing technologies or developing new, our goal is simple, to accelerate next generation products to market, advancing a smoke-free future.
What is the Blue-sky thinking hub?
In nicotine, pharmaceutical, and cannabis industries there are current and future global industry challenges to achieve total harm reduction and wellbeing targets. To achieve short-term goals and ensure sustainability for the future, we need to act together now to make a difference. Our open innovation community is based on value creation through collaboration to build sustainable partnerships within these industries. Knowledge inputs and outputs are invaluable to accelerate innovation to advance future technologies in an agile environment.
What's your innovation?
If you have an idea in any of the following areas, we would like to hear from you:
- Novel active delivery
- Formulations
- Plastics
- Heating
- Technology
- Power
- Regulations
Innovate to accelerate!
Through collaboration, wouldn't it be fantastic to develop the next big thing, whatever guise that may be in. Developing new technologies in areas such as software applications, environmentally friendly plastics, alternative power sources to batteries, sustainable packaging, and novel routes of delivery will deliver ground-breaking advances in science. Who knows – we could even help influence/shape future policy. We welcome expert knowledge from around the globe to stimulate healthy debate amongst industry professionals. Let's collaborate, innovate, and accelerate the pathway to success - together we can make a difference.
Learn more by clicking the box below:
Can we help you?
Broughton have deep CRO capabilities and an experienced team of scientific and regulatory professionals to manage full service global regulatory projects. Our knowledge of global regulatory pathways offers significant insights that can be incorporated into your project plans. Our mission is to accelerate safer nicotine-delivery products to market; advancing a smoke-free future.
Book a meeting with us today to discuss your requirements.



